Home
> “The origin of SARS-CoV-2 is being seriously questioned”
“The origin of SARS-CoV-2 is being seriously questioned”
11.09.2020,
by
Yaroslav Pigenet
Nearly a year after the SARS-CoV-2 coronavirus was
identified, researchers have yet to determine how it
“jumped species” to infect humans. Virologist Étienne
Decroly discusses the various hypotheses, including that
of an accidental leak from a laboratory.
At a time when researchers are racing against
the clock to develop viable vaccines and treatments,
why is it so important to understand the genealogy of
the virus behind the Covid-19 pandemic?
Étienne Decroly: After
SARS-CoV in 2002 and MERS-CoV in 2012, SARS-CoV-2, which
was quickly identified as causing Covid-19, is the third
human coronavirus responsible for a severe respiratory
syndrome to have emerged in the past 20 years. We are
now quite familiar with this family of viruses, which
circulate primarily among bats, and whose zoonotic
transfer occasionally triggers epidemics among humans.
It is therefore crucial to understand how this pathogen
crossed the species barrier and became easily
transmissible from human to human. It is essential to
study the evolutionary mechanisms and molecular
processe s
involved in the advent of this pandemic virus in order
to better anticipate potential outbreaks of this type,
and to develop therapeutic and vaccinal strategies.
In the early weeks of the pandemic, when we
knew very little about the virus, it was very quickly
suspected to be of animal origin. Why was this
possibility immediately favoured, and has it since
been confirmed?
É.D.: The zoonotic origin of
coronaviruses, which infect nearly 500 species of bat,
was already well documented from previous outbreaks. In
nature, different bat populations share the same caves,
and various viral strains can contaminate the same
animal simultaneously. This situation facilitates
genetic recombination between viruses and their
evolution, allowing certain strains to develop the
capacity to cross the species barrier.
[...img...]
Genome sequence comparisons of viral samples from different
patients infected by SARS-CoV-2 have revealed an identity rate of
99.98%, indicating that the strain emerged in humans very
recently. It was also soon discovered that this genome is 96%
identical to that of a bat virus (RaTG13) collected in 2013 from
the animals’ guano, whose sequences have only been known since
March 2020. In addition, one fragment of this genome proved to be
totally identical to another, made up of 370 nucleotides,
sequenced in 2016 from samples collected in 2013 at a mine in
China’s Yunnan province where three miners had died of severe
pneumonia.
Furthermore, analyses of other known human coronaviruses show only
79% genetic identity between SARS-CoV-1 and SARS-CoV-2, and only
50% for MERS-CoV. Simply put, SARS-CoV-2 is genetically closer to
virus strains that were previously transmitted only among bats. It
did not descend from known human strains and only recently
acquired the ability to leave its natural animal reservoir, which
is most likely bats.
If it has been determined that Covid-19 came from bats,
why is there still such controversy over its origins?
É.D.: Since no case of an epidemic caused by
direct bat-to-human transmission has yet been demonstrated, it is
thought that the transfer to humans more probably took place via
an intermediate host species in which the virus could evolve and
move towards forms likely to infect human cells. Such an
intermediary is usually identified by examining the phylogenetic
relations between the new virus and those that contaminate animal
species living near the outbreak zone. This method made it
possible to determine that the civet was probably the secondary
host of SARS-CoV in the early 2000s, and the dromedary that of
MERS-CoV ten years later. The discovery, in the genome of a
coronavirus infecting pangolins, of a short genetic sequence
coding for the recognition domain of receptor ACE-2, related to
the sequence that allows SARS-CoV-2 to penetrate human cells,
first suggested that a possible intermediary had been found, but
the rest of its genome is too dissimilar to SARS-CoV-2 to be a
direct ancestor.
[img]
SARS-CoV-2 could thus have resulted from multiple recombinations
among different coronaviruses circulating in pangolins and bats,
leading to an adaptation that enables transmission to humans. In
this case, a secondary cause of the Covid-19 pandemic would have
been contact with the intermediate host, possibly an animal sold
in the market in Wuhan (China). However, this hypothesis raises
many questions. First of all, the geography: the viral samples
from bats were collected in Yunnan, nearly 1,500 kilometres from
Wuhan, where the pandemic began. There is also an ecological
issue: bats and pangolins inhabit different ecosystems, so it is
difficult to imagine how their viruses could have recombined. Most
importantly, it has been noted that the identity rate between the
SARS-CoV-2 sequences and those from pangolins reaches a mere
90.3%, which is far lower than what is normally observed between
strains infecting humans and those contaminating secondary hosts.
The genomes of SARS-CoV and the civet strain from which it
descended, for example, are 99% identical.
Could you tell us more about the cellular receptor’s
recognition sequence and the mechanism that allows the virus to
penetrate cells?
É.D.: That has to do with the biological
characteristics of coronaviruses. Their genome contains an S gene
coding for the spike protein, which enters into the composition of
the envelope and gives the coronavirus its characteristic
“crown” shape. The spike protein plays a fundamental role in the
virus’s infection capacity because it contains a domain, called
RBD, which has the property of binding specifically to certain
receptors (ACE2) on the surface of infectible cells. It is the
establishment of this link that then allows the pathogen to
penetrate the cell. The RBD domain’s affinity for ACE2 receptors
in a given species is a determining factor in the virus’s
infection capacity for that species. In humans, this receptor is
widely expressed and can be found, for example, on the surface of
pulmonary and intestinal cells.
[img]
Analyses of coronavirus databases have made it possible to
determine that the genetic sequence coding for the RBD domain of
SARS-CoV-2 is very close to that of the coronavirus infecting
pangolins. This observation suggests that the spike protein of the
CoV infecting these animals has a strong affinity for the human
ACE2 receptor, which possibly enabled that pathogen to enter human
cells more easily than the bat virus. However, for reasons
mentioned above, most researchers now think that the pangolin
probably played no role in the emergence of SARS-CoV-2. The
prevalent hypothesis today is that it was more likely a
convergent, independent evolution of the RBD domain in both virus
strains.
Are there any indications of other candidates for the
role of intermediate host?
É.D.: In zoonoses, secondary hosts are
usually found among livestock or wild animals that come into
contact with the human population. In this case, despite
research on viruses found in the animal species sold at the
Wuhan market, no intermediary virus between RaTG13 and
SARS-CoV-2 has been singled out so far. Until one is identified
and its genome sequenced, the question of the origin of
SARS-CoV-2 will remain unanswered. For lack of convincing
evidence concerning the last animal intermediary before human
contamination, some sources are suggesting that the virus could
have crossed the species barrier following a laboratory accident
or even be man-made
[img].
Do you think that SARS-CoV-2 escaped from a laboratory?
É.D.: The hypothesis cannot be ruled out,
given that SARS-CoV, which emerged in 2003, has escaped from
laboratory experiments at least four times. In addition, there’s
the fact that coronaviruses were a major area of study in the
laboratories near the SARS-CoV-2 outbreak zone, where researchers
were investigating, among other things, the mechanisms involved in
crossing the species barrier. However, at this time, the analyses
based on the phylogeny of the complete virus genomes yield no
clear conclusions on the evolutionary origin of SARS-CoV-2.
There are three main scenarios for explaining how the latter
acquired its epidemic potential. First of all, it is a zoonosis.
Covid-19 is caused by the recent breaching of the species barrier
by a coronavirus. In this case, there must be another virus with
greater similarity than RaTG13 in a domestic animal or livestock
species, but, as previously mentioned, no such strain has yet been
found.
The second scenario is that it could be a coronavirus different
from SARS-CoV or MERS-CoV that adapted to humans several years ago
and circulated relatively unnoticed until a recent mutation made
it more transmissible from an individual to another. To confirm
this hypothesis, we would have to analyse virus samples from
people who died of atypical pneumonias in the outbreak zone before
the pandemic broke out. Lastly, SARS-CoV-2 may have
descended from a bat virus isolated by scientists collecting
samples, which then adapted to other species during research on
animal models in the laboratory – laboratory from which it then
accidentally escaped.
Isn’t there a risk that this last hypothesis may uphold
the conspiracy theories about the Covid-19 pandemic?
É.D.: Studying the origin of SARS-CoV-2 is a
scientific process that cannot be equated with a conspiracy
theory. At the same time, I would like to underline the fact that,
as long as no intermediate host has been identified, the
scientific community cannot rule out the possibility of an
accidental leak.
As of today, no scientific study has produced any clear evidence
to confirm this. Nonetheless, the fact remains that further
analyses are needed to reach a conclusion. The question of the
natural or synthetic origin of SARS-CoV-2 cannot be made
contingent on a political agenda or communication strategy. It
deserves to be examined in light of the scientific data at our
disposal.
Our hypotheses must also take into account what virology
laboratories are capable of doing at this stage, and the fact that
the manipulation of potentially pathogenic virus genomes is a
common practice in certain laboratories, in particular for
studying how viruses cross the species barrier.
Indeed, many conspiracy websites echo the assertions of
Luc Montagnier, who explained that SARS-CoV-2 is a “chimera
virus” created in a Chinese laboratory, a cross between a
coronavirus and the human immunodeficiency virus (HIV). Is this
a serious theory?
É.D.: In any case, it is no longer taken
seriously by specialists, who have refuted its main conclusions.
Nonetheless, it is based on an utterly serious observation that is
important for understanding the infection mechanism of SARS-CoV-2:
it has been discovered that the gene coding for the spike protein
contains four insertions of short sequences that are not found in
the most genetically similar human coronaviruses. These insertions
probably give the spike protein of SARS-CoV-2 exceptional
properties. Structural studies indicate that the first three
insertions are located on exposed domains of the S protein and are
thus likely to play a role in how the virus evades the host’s
immune system.
The fourth insertion, which is more recent, produces a site
sensitive to furins, protease enzymes produced by the host cells.
It has now been clearly demonstrated that furin cleavage of the
spike protein induces a conformational change that is conducive to
the recognition of the ACE2 cellular receptor. Researchers
investigating the origin of these insertions have reported in a
pre-publication that these sequences of the SARS-CoV-2 spike
protein show unsettling similarities with fragment sequences of
the HIV-1 virus. Strongly criticised for its methodological
shortcomings and errors of interpretation, the article was deleted
from the bioRxiv site.
[img]
This postulate would have remained insignificant, had it not been
revived by Luc Montagnier, winner of the Nobel Prize in Physiology
or Medicine for his work on HIV. In April 2020, he claimed that
these insertions did not result from natural recombination nor
occurred accidentally, but from deliberate gene manipulation,
probably in the course of research to develop HIV vaccines. These
assertions were once again refuted by biostatistical analyses,
which showed that the similar sequences in HIV and SARS-CoV-2 are
too short (10 to 20 nucleotides out of a total of 30,000 for the
genome) and that the resemblance is most likely coincidental.
Meanwhile, faced with the difficulty of understanding the origin
of this pathogen, we have conducted phylogenetic
analyses in collaboration with bioinformaticians and
phylogeneticists .
Their findings show that three of the four insertions observed in
SARS-CoV-2 can be found in older coronavirus strains. Our study
clearly shows that these sequences appeared independently, at
different times in the evolutionary history of the virus. This
data invalidates the hypothesis of a recent and intentional
insertion by a laboratory of those three sequences.
That leaves the fourth insertion, which produces a furin protease
cleavage site in SARS-CoV-2 that is not found in the other viruses
of the SARS-CoV family. Consequently, the possibility cannot be
ruled out that this insertion results from experiments designed to
allow an animal virus to jump species to humans, since it is well
known that this type of insertion plays a key role in the
propagation of many pathogens in humans.
How can we know for sure?
É.D.: The SARS-CoV-2 genome is a combinatory
puzzle and the recombination mechanisms of the animal viruses that
led to its emergence remain a mystery. To understand its genesis,
many more samples from wild and domestic species need to be
collected. The possible discovery of animal diseases with a very
strong similarity to SARS-CoV-2 would be a key element for
confirming its natural origin. In addition, more in-depth
bioinformatic analyses could reveal possible traces of genetic
manipulation, which would conversely suggest an experimental
origin.

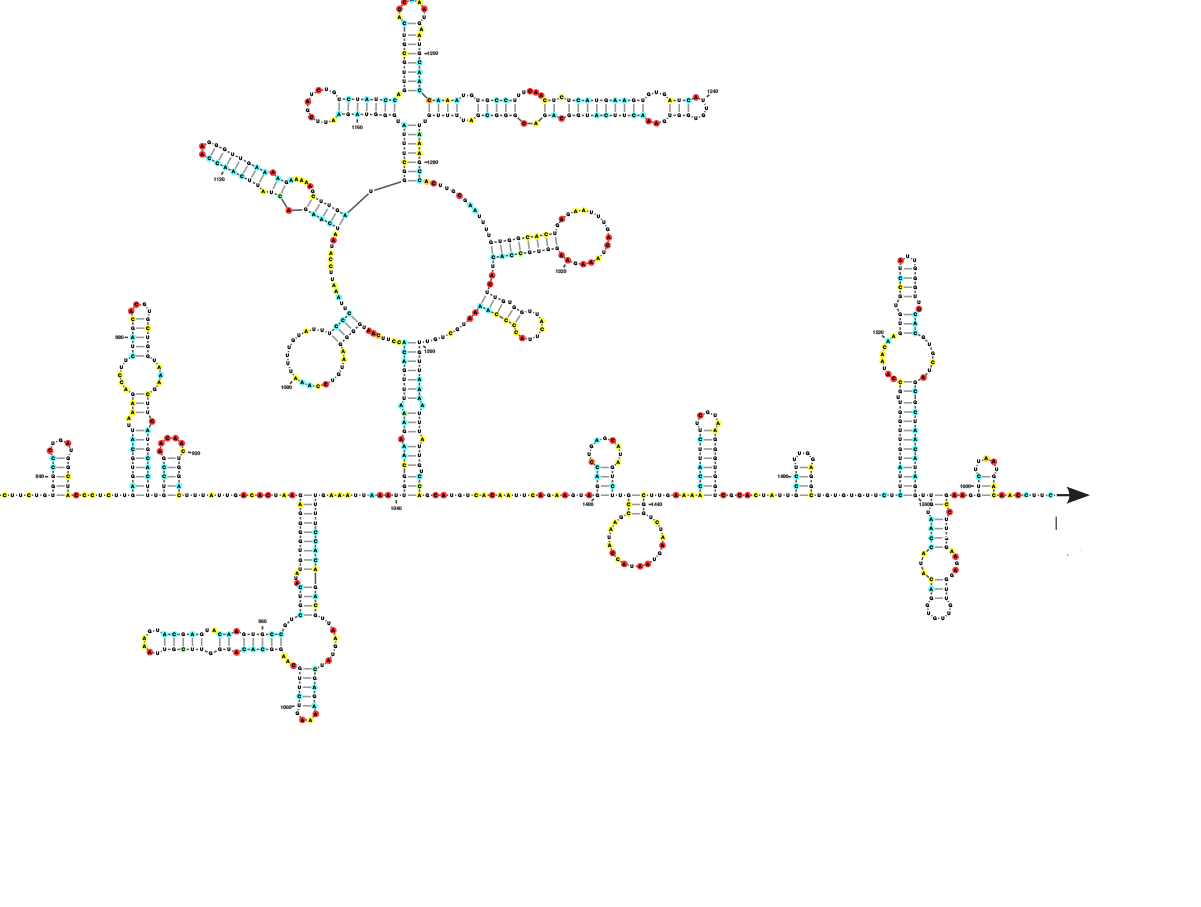
Diagrammatic representation of
part of the SARS-CoV-2 genome.
Tammy C. T. Lan et al.,
bioRxiv; S. Rouskin/Boston University
In any case, whether the virus is natural or not, the very fact
that this question can now be seriously considered calls for a
critical review of the reconstruction tools and methods being used
in today’s research laboratories, and of their potential use in
“gain-of-function” experiments.
But aren’t those the only tools that can help us to
understand and combat these viruses and the epidemics they
cause?
É.D.: Indeed, but we must understand that
the paradigms of virus research have changed radically in recent
years. Today, any laboratory can obtain or synthesise a gene
sequence. It’s possible to build a functional virus from scratch
in less than a month using sequences available in the databases.
In addition, gene manipulation tools have been developed that are
fast, easy to use and inexpensive. They enable spectacular
progress, but at the same time multiply the risk and possible
severity of an accident, in particular in gain-of-function
experiments on viruses with pandemic potential.
Even if it ultimately turns out that the Covid-19 epidemic is the
result of a “classic” zoonosis, incidents of pathogens escaping
from laboratories have been documented in recent years. One of the
best-known cases is the Marburg virus disease, which originated
from contamination by wild monkeys. The 1977 flu pandemic is
another example. Recent genetic studies suggest that it was caused
by the leak of a virus strain, collected in the 1950s, from a
laboratory. More recently, several such accidental leaks from
studies of SARS-CoV have been reported in the literature.
Fortunately, none of them caused a major epidemic.
[img]
International standards require that any research,
isolation or culturing involving potentially pandemic
viruses, including respiratory ones, must be conducted
under secure experimental conditions, with
irreproachable traceability in order to prevent any
zoonotic transmission. However, accidents can always
happen. It is important to consider the potential risk
of such experiments, especially if they target gain of
function or infectivity.
Are you in favour of a moratorium or ban on
this type of research?
É.D.: I do not advocate an outright
ban. The point is not to “sterilise” research, but to
examine the benefit-to-risk ratio more rigorously.
Perhaps a conference should be organised to evaluate the
need for a moratorium or more suitable international
regulation.
Considering the risks of infection arising from the
techniques used in virus research today, civil society
and the scientific community must urgently re-examine
the practice of gain-of-function experiments and the
artificial adaptation of viral strains in intermediary
animal hosts. In 2015, aware of this problem, the
federal agencies in the United States froze funding for
all new studies involving this type of experiment. The
moratorium ended in 2017. In my opinion, these high-risk
practices should be reconsidered, and monitored by
international ethics committees.
Lastly, researchers in these fields must also be more
sensitive to their own responsibility whenever they are
conscious of the possible dangers incurred by their
work. There are often alternative experimental
strategies that can achieve the same purpose while
greatly reducing the risks.
Aren’t those strategies already used?
É.D.: In theory, yes. In reality,
we often fall short of the goal, especially because we
scientists do not receive sufficient training on these
issues. And because the climate of competition that
reigns in the world of research encourages fast, frantic
experimentation that does not really take ethical
questions into account, nor weigh a project’s potential
risks.
In the master’s programme that I teach on viral
engineering, I have been giving, for about ten years, a
theoretical exercise which consists in imagining a
process that would give HIV the capacity to infect any
cell in the body (and not just lymphocytes). While most
of the students are able to come up with an effective
method for building a potentially dangerous chimera
virus, they focus exclusively on the effectiveness of
the technique, without ever questioning the potential
consequences of its implementation.
My goal here as a teacher is to make them aware of the
issues involved and show them that in many cases it is
possible to build experimental systems that are just as
effective but offer better control of the biological
risks. Starting early in the educational process, we
need to train future biologists to always assess the
risk and social relevance of their research, however
innovative it may be.
Comments
Links:
[1] https://news.cnrs.fr/
[2]
https://lejournal.cnrs.fr/articles/la-question-de-lorigine-du-sars-cov-2-se-pose-serieusement
[3] https://news.cnrs.fr/life
[4] https://news.cnrs.fr/virology-0
[5] https://news.cnrs.fr/javascript%3A%3B
[6] https://news.cnrs.fr/authors/yaroslav-pigenet
[7] https://hal.archives-ouvertes.fr/hal-02891455
[8]
https://twitter.com/intent/tweet?status=https%3A//news.cnrs.fr/print/1648
[9]
http://www.facebook.com/sharer/sharer.php?s=100&p%5Burl%5D=https%3A//news.cnrs.fr/print/1648&p%5Btitle%5D=%E2%80%9CThe%20origin%20of%20SARS-CoV-2%20is%20being%20seriously%20questioned%E2%80%9D&p%5Bimages%5D%5B0%5D=https%3A//news.cnrs.fr/sites/default/files/styles/lightbox-hd/public/assets/images/000_1ph6hc_72dpi.jpg%3Fitok%3D-Aa6HQMO&p%5Bsummary%5D=
[10]
https://plus.google.com/share?url=https%3A//news.cnrs.fr/print/1648
[11]
http://www.facebook.com/sharer/sharer.php?s=100&p%5Burl%5D=https%3A//news.cnrs.fr/print/1648&p%5Btitle%5D=%E2%80%9CThe%20origin%20of%20SARS-CoV-2%20is%20being%20seriously%20questioned%E2%80%9D&p%5Bimages%5D%5B0%5D=https%3A//news.cnrs.fr/sites/default/files/styles/lightbox-hd/public/assets/images/000_aph2003052646462_72dpi.jpg%3Fitok%3D4XqWV1Fu&p%5Bsummary%5D=
[12]
http://www.facebook.com/sharer/sharer.php?s=100&p%5Burl%5D=https%3A//news.cnrs.fr/print/1648&p%5Btitle%5D=%E2%80%9CThe%20origin%20of%20SARS-CoV-2%20is%20being%20seriously%20questioned%E2%80%9D&p%5Bimages%5D%5B0%5D=https%3A//news.cnrs.fr/sites/default/files/styles/lightbox-hd/public/assets/images/virus_and_ace2_yellow_8k_nolabel_72dpi.png%3Fitok%3DNhWBfApr&p%5Bsummary%5D=
[13]
http://www.facebook.com/sharer/sharer.php?s=100&p%5Burl%5D=https%3A//news.cnrs.fr/print/1648&p%5Btitle%5D=%E2%80%9CThe%20origin%20of%20SARS-CoV-2%20is%20being%20seriously%20questioned%E2%80%9D&p%5Bimages%5D%5B0%5D=https%3A//news.cnrs.fr/sites/default/files/styles/lightbox-hd/public/assets/images/bios-2171803_72dpi.jpg%3Fitok%3D8R3KJwXp&p%5Bsummary%5D=
[14]
http://www.facebook.com/sharer/sharer.php?s=100&p%5Burl%5D=https%3A//news.cnrs.fr/print/1648&p%5Btitle%5D=%E2%80%9CThe%20origin%20of%20SARS-CoV-2%20is%20being%20seriously%20questioned%E2%80%9D&p%5Bimages%5D%5B0%5D=https%3A//news.cnrs.fr/sites/default/files/styles/lightbox-hd/public/assets/images/protein_with_glycans_ieee_72dpi.png%3Fitok%3DSRPjsa9J&p%5Bsummary%5D=
[15]
https://www.sciencedirect.com/science/article/pii/S0166354220300528
[16]
http://www.facebook.com/sharer/sharer.php?s=100&p%5Burl%5D=https%3A//news.cnrs.fr/print/1648&p%5Btitle%5D=%E2%80%9CThe%20origin%20of%20SARS-CoV-2%20is%20being%20seriously%20questioned%E2%80%9D&p%5Bimages%5D%5B0%5D=https%3A//news.cnrs.fr/sites/default/files/styles/lightbox-hd/public/assets/images/000_u23yf_72dpi.jpg%3Fitok%3DNEooO2FJ&p%5Bsummary%5D=
[17]
http://www.facebook.com/sharer/sharer.php?s=100&p%5Burl%5D=https%3A//news.cnrs.fr/print/1648&p%5Btitle%5D=%E2%80%9CThe%20origin%20of%20SARS-CoV-2%20is%20being%20seriously%20questioned%E2%80%9D&p%5Bimages%5D%5B0%5D=https%3A//news.cnrs.fr/sites/default/files/styles/lightbox-hd/public/assets/images/supplementaryfigure1-1_72dpi.png%3Fitok%3DzVdqiYZ9&p%5Bsummary%5D=
[18]
http://www.facebook.com/sharer/sharer.php?s=100&p%5Burl%5D=https%3A//news.cnrs.fr/print/1648&p%5Btitle%5D=%E2%80%9CThe%20origin%20of%20SARS-CoV-2%20is%20being%20seriously%20questioned%E2%80%9D&p%5Bimages%5D%5B0%5D=https%3A//news.cnrs.fr/sites/default/files/styles/lightbox-hd/public/assets/images/000_ly8tq_72dpi.jpg%3Fitok%3DEG7nkTSu&p%5Bsummary%5D=
[19] https://news.cnrs.fr/sars-cov-2
[20] https://news.cnrs.fr/covid-19-1
[21] https://news.cnrs.fr/hiv
[22] https://news.cnrs.fr/phylogeny
[23] https://news.cnrs.fr/vaccine
[24] https://news.cnrs.fr/virus-0
[25] https://news.cnrs.fr/laboratories
[26] https://news.cnrs.fr/pangolin
[27] https://news.cnrs.fr/bat
[28] https://news.cnrs.fr/species
[29] https://news.cnrs.fr/barrier
[30] https://news.cnrs.fr/protein
[31] https://news.cnrs.fr/spike
[32] https://news.cnrs.fr/genome-0
[33] https://news.cnrs.fr/toxicity
[34] https://news.cnrs.fr/sars
[35] https://news.cnrs.fr/domain
[36] https://news.cnrs.fr/ace2-receptors
[37]
http://www.facebook.com/sharer/sharer.php?s=100&p%5Burl%5D=https%3A//news.cnrs.fr/print/1648&p%5Btitle%5D=%E2%80%9CThe%20origin%20of%20SARS-CoV-2%20is%20being%20seriously%20questioned%E2%80%9D&p%5Bimages%5D%5B0%5D=&p%5Bsummary%5D=
[38] https://news.cnrs.fr/printmail/1648
[39]
https://news.cnrs.fr/articles/a-capsule-to-explore-the-intestine
[40]
https://news.cnrs.fr/articles/social-inequality-impacts-stroke-recovery
[41]
https://news.cnrs.fr/articles/invasive-species-an-ecological-and-economic-disaster
[42]
https://news.cnrs.fr/slideshows/keeping-an-eye-on-the-forest
[43]
https://news.cnrs.fr/slideshows/landscapes-of-the-microworld
[44]
https://news.cnrs.fr/articles/researchers-step-up-the-fight-against-covid-19
[45]
https://news.cnrs.fr/articles/a-century-on-does-bcg-have-a-future
[46]
https://news.cnrs.fr/articles/covid-19-variants-are-a-game-changer
[47] https://news.cnrs.fr/articles/which-vaccines-for-covid-19
[48]
https://news.cnrs.fr/articles/virology-between-a-marathon-and-a-race-against-time
[49]
https://news.cnrs.fr/articles/chemists-in-the-gavo-project-set-out-to-tackle-viruses
[50]
https://news.cnrs.fr/articles/bioinformatics-a-key-ally-in-the-fight-against-covid-19
[51]
https://news.cnrs.fr/articles/is-ventilation-the-ultimate-weapon-against-covid-19
[52]
https://news.cnrs.fr/opinions/do-covid-19-vaccines-prevent-infection-and-transmission
[53] https://news.cnrs.fr/crss/node/1648
[54] https://news.cnrs.fr/user/login?destination=print/1648
[55] https://news.cnrs.fr/user/register?destination=print/1648



Log in, join the CNRS News community